Arbeitsgruppe | Prof. Bauersachs | Dr. Fraccarollo | Dr. Galuppo
Research Focus
The topic of our research is cardiac wound healing and remodeling after ischemia.
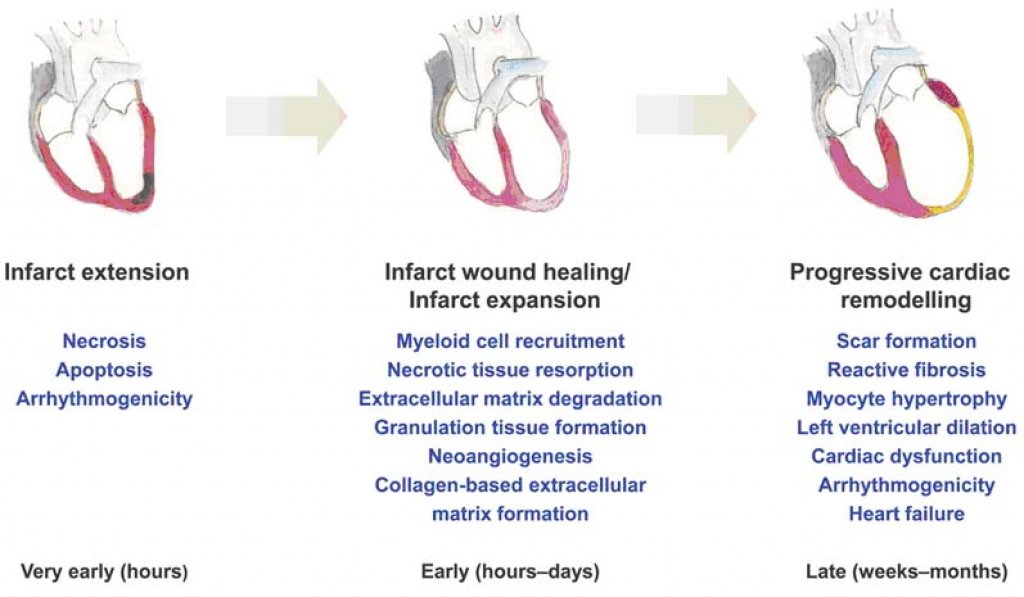
Ischemic cell death and the subsequent loss of contractile myocardium trigger a cascade of cellular events that promote infarct wound healing and dynamic time-dependent structural changes of the infarcted region and the residual viable myocardium. Thinning and dilatation of the infarcted region (infarct expansion) are events of the early phase of healing, followed by progressive architectural rearrangements of the surviving myocardium, involving extracellular matrix remodeling, cardiomyocyte hypertrophy, as well as dilatation and distortion of ventricular shape.Our group provided important insights into the mechanisms underlying cardioprotection by endothelin receptor antagonism, HMG-CoA-reductase inhibition, restoration of impaired NO-sGC-cGMP signaling as well as mineralocorticoid receptor-blocking therapy in experimental ischemic heart failure. Recent data, involving mice with cardiomyocyte-restricted inactivation of the mineralocorticoid receptor gene showed that the clinical benefits of MR blocking therapy in myocardial infarction and heart failure are mediated largely via cardiomyocyte-dependent mechanisms, and provided strong evidence that more favorable effects on cardiac dysfunction and failure can be achieved by early initiation of mineralocorticoid receptor blockade postinfarction.We are analysing cellular and molecular mechanisms regulanting infarct wound healing and cardiac remodeling after myocardial ischemia. Our scientific goal is the development of novel therapeutic strategies that limit ischemia/reperfusion injury and enhance wound healing/repair of damaged myocardium to prevent progressive ventricular dilation, functional deterioration and heart failure. A major research focus is to elucidate the pathophysiological relevance of the adrenocortical steroid receptors in myeloid cells for wound healing after myocardial ischemia.
-
Fraccarollo D, Galuppo P, Bauersachs J. Novel therapeutic approaches to post-infarction remodelling. Cardiovasc Res. 2012 ;94:293-303.
Fraccarollo D, Bauersachs J. Cardiomyocyte mineralocorticoid receptor function post myocardial infarction. Trends Cardiovasc Med. 2012;21:42-7.
-
Fraccarollo D, Berger S, Galuppo P, Kneitz S, Hein L, Schutz G, Frantz S, Ertl G, Bauerschs J. Deletion of cardiomyocyte mineralocorticoid receptor ameliorates adverse remodeling after myocardial infarction. Circulation 2011;123:400-8.
-
Fraccarollo D, Widder JD, Galuppo P, Thum T, Tsikas D, Hoffmann M, Bauersachs J. Improvement in left ventricular remodeling by the endothelial nitric oxide synthase enhancer AVE9488 after experimental myocardial infarction. Circulation 2008;118:818-27.
-
Fraccarollo D, Galuppo P, Schraut S, Kneitz S, van Rooijen N, Ertl G, Bauersachs J. Immediate mineralocorticoid receptor blockade improves myocardial infarct healing by modulation of the inflammatory response. Hypertension 2008;51:905-14.
-
Fraccarollo D, Galuppo P, Schmidt I, Ertl G, Bauersachs J. Additive amelioration of left ventricular remodeling and molecular alterations by combined aldosterone and angiotensin receptor blockade after myocardial infarction. Cardiovasc Res 2005;67:97-105.
-
Fraccarollo D, Galuppo P, Hildemann S, Christ M, Ertl G, Bauersachs J. Additive improvement of left ventricular remodeling and neurohormonal activation by aldosterone receptor blockade with eplerenone and ACE inhibition in rats with myocardial infarction. J Am Coll Cardiol 2003;42:1666-73
-
Fraccarollo D, Bauersachs J, Kellner M, Galuppo P, Ertl G. Cardioprotection by long-term ET(A) receptor blockade and ACE inhibition in rats with congestive heart failure: mono- versus combination therapy. Cardiovasc Res 2002;54:85-94.
-
Fraccarollo D, Galuppo P, Bauersachs J, Ertl G. Collagen accumulation after myocardial infarction: effects of ETA receptor blockade and implications for early remodeling. Cardiovasc Res 2002;54:559-67.
-
Bauersachs J, Galuppo P, Fraccarollo D, Christ M, Ertl G. Improvement of left ventricular remodeling and function by hydroxymethylglutaryl coenzyme a reductase inhibition with cerivastatin in rats with heart failure after myocardial infarction. Circulation 2001;104:982-5.
-
Bauersachs J, Fraccarollo D, Ertl G, Gretz N, Wehling M, Christ M. Striking increase of natriuresis by low-dose spironolactone in congestive heart failure only in combination with ACE inhibition: mechanistic evidence to support RALES. Circulation. 2000;102(19):2325-8
-
Fraccarollo D, Hu K, Galuppo P, Gaudron P, Ertl G. Chronic endothelin receptor blockade attenuates progressive ventricular dilation and improves cardiac function in rats with myocardial infarction: possible involvement of myocardial endothelin system in ventricular remodeling. Circulation 1997;96:3963-73.
-
Determination of total and mitochondrial superoxide anion formation in cells (monocytes, adult/neonatal cardiomyocytes, fibroblasts, endothelial cells), tissue (heart, aorta) by HPLC-Electrochemical analysis of mito-2-hydroxy-E+ and 2-hydroxy-E+
-
Direct determination of tetrahydrobiopterin, dihydrobiopterin, and biopterin by HPLC using sequential electrochemical and fluorescence detection in plasma, tissue (heart, aorta), isolated cardiac cells
-
Simultaneous quantification of reduced and oxidized glutathione in plasma, tissue (heart, aorta), isolated cardiac cells
-
Collagen turnover analysis (MMP/TIMP activity, hydroxyproline, peptide mapping of collagen chains)
-
NF-κB DNA binding activity in isolated cardiac cells
-
Receptor binding assay
-
Determination of catecholamine and metabolites, adenine nucleotides, collagen turnover markers, cytokines/chemokines, neurohormones (aldosterone, corticosterone/cortisol, FAP, NTproANP/BNP, PRA, endothelin, angiotensin II) in plasma/tissue by HPLC-Electrochemical/Fluorescence/UV detection, ELISA, RIA, multiplex assays
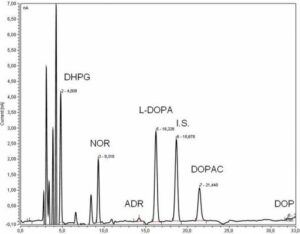
Determination of catecholamine and metabolites in human plasma (300 µL) by HPLC-EC.jpg

Determination of biopterins by HPLC using sequential electrochemical (EC) – BH4 – and fluorescence (FL) – BH2 and biopterin- detection in diabetic mouse aorta
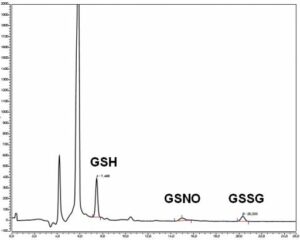
HPLC-EC analysis of glutathione, nitrosoglutathione and glutathione disulfide in mouse (myo)fibroblasts isolated from ischemic myocardium and exposed to hypoxia
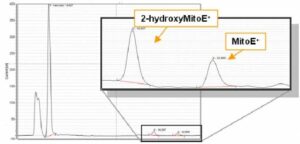
HPLC-EC analysis of Mito-2-hydroxy-E+ in mouse blood monocytes (CD115+) 24 hours after myocardial ischemia
Immunophenotyping
-
Evaluation of monocyte, lymphocyte and mesenchymal subsets in human blood by 7-color flow cytometry panels
-
Characterization of mouse neutrophil phenotypes, dendritic cells, endothelial cells, monocyte/macrophage subsets in blood, ischemic/surviving myocardium, spleen, bone marrow, peritoneum by multicolor color flow cytometry
-
Characterization of macrophage polarization, fibroblast/myofibroblast differentiation of cells isolated from stressed hearts (ischemia, overload) by multicolor flow cytometry and immunocytochemical staining.
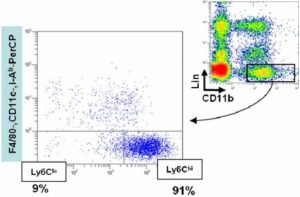
Characterization of mouse monocyte/macrophage subsets in the healing myocardium and blood 2 days after ischemia
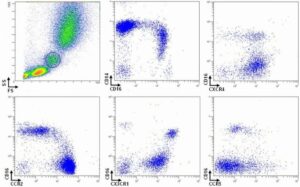
Immunophenotypic characterization of human blood monocyte subsets
Gene expression profiling
-
RNA, DNA quality assessment by Agilent 2100 Bioanalyzer and NanoDrop 2000c
-
Real-time multiplex assay
-
RNA/microRNA microarray data analysis by R software and hierarchical clustering explorer (HCE)
-
Graphical visualization: hierarchical clustering, heatmap, MA/volcano plot, principal component analysis (PCA)
-
Gene Set Enrichment Analysis (GSEA)
-
Gene Map Annotator and Pathway Profiler (GenMAPP)
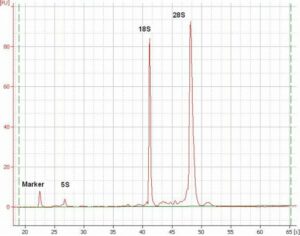
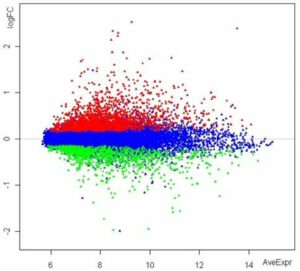
RNA analysis with Agilent 2100 Bioanalyzer. Microarray analysis showing up- (red), down-regulated (greerTMN Ruhn) genes
Multivariate statistical data analysis and visualisation
- Programming in R, language and environment for statistical computing and graphics
Cardiovascular phenotyping
Models of cardiac injury in mouse/rat
- Myocardial infarction,
- Ischemia/Reperfusion
- Coronary artery stenosis
- Angiotensin II, aldosterone induced hypertension
- Monocyte depletion
- Streptozotocin induced diabetes
- LV pressure-volume loops measured in vivo using Millar conductance catheter SPR-839 in mice 8 weeks after myocardial ischemia or sham operation

Transient ischemia
Permanent ischemia
Hemodynamics and vascular reactivity analysis in mouse / rat
- In vivo left ventricular pressure-volume measurements during steady state/vena cava occlusion
- Cardiac contractility/relaxation analysis during ß-adrenergic stimulation
- Measurement of isometric force in vascular rings
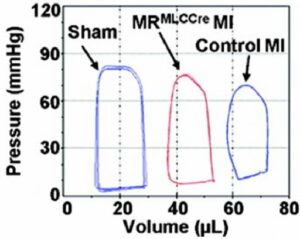
LV pressure-volume loops measured in vivo using Millar conductance catheter SPR-839 in mice 8 weeks after myocardial ischemia or sham operation
Histology and histochemistry
- In vivo heart perfusion/fixation, paraffin/Tissue Tek embedding, section cutting
- In situ apoptosis detection by TACSTM Blue Label
- Triple immunofluorescence staining, dual immunohistochemical staining, image processing with NIS-elements NIKON
- Determination of infarct extension/expansion/size, collagen content/structural organization
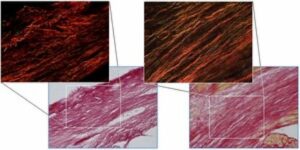
Sirius Red polarization microscopy (enlarged insets) of scar sections 7 days after myocardial ischemia
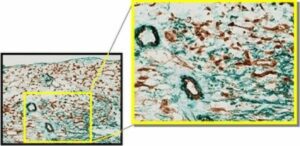
Pericyte-coated vessels and myofibroblasts (identified by α-smooth muscle actin immunoistochemistry, green) and capillaries (CD31 staining, brown) in the healing myocardium 7 days after ischemia
Isolation, culture/coculture, characterization of adult/neonatal mouse/rat cardiac cells
Cell culture models of hypoxia and acidosis
Analysis of migration, invasion, phagocytosis by fluorescence microscopy and fluorometric assays
- Cardiomyocytes
- Fibroblasts
- Endothelial cells
- Myeloid cells
- -Progenitor cells

Mouse adult cardiomyocytes
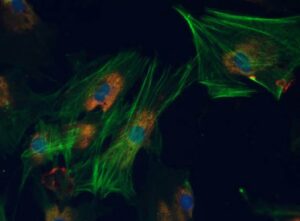
(Myo)fibroblasts: Immunocytochemical staining showing α-SMA (green)and collagen (red)
Group leader
Kardiologisches Forschungslabor
Gebäude I3
Ebene SO
Raum 2290
Tel: 0511 532-5772 | 0511 532-5773
Fax: 0511 532-8194
Dr. Daniela Fraccarollo
fraccarollo.daniela@mh-hannover.de
Dr. Paolo Galuppo
galuppo.paolo@mh-hannover.de

